Real World Evidence (RWE) refers to data collected from sources outside of traditional randomized controlled trials (RCTs), such as patient registries, electronic health records, and observational studies. RWE helps us understand how treatments work in everyday clinical settings, providing insights into patient outcomes, adherence, and treatment effectiveness across diverse populations.
While RCTs are essential for establishing clinical efficacy under controlled conditions, RWE provides a broader understanding of how therapies perform in the real world, including patient groups often excluded from clinical trials. This data is particularly valuable for diseases like Axial Spondyloarthritis (AS) and Rheumatoid Arthritis (RA), where treatment decisions are based on individual patient needs and real-world experiences.
But why are we looking at RWE and registry data, not RCTs only?
Only 7.6 - 44% of the patients would have been potentially eligible for the RCTs.10
RCT: randomized controlled trial
RWE Persistence in mixed populations
In a systematic review and pooled data analysis from 27 European immune-mediated rheumatic disease (IMRD) studies, a total of 12 studies reported comparative persistence data of Simponi® with other biological treatments. Simponi® was reported to have significantly higher persistence than ADA in seven studies, whereas two studies found no significant difference between the two comparators. Compared with CZP, four studies found no significant difference in persistence, whereas four other studies found that Simponi® had a significantly higher persistence.31
Four studies reported effect size of the difference in persistence, formulated as HRs, using Simponi® as the reference treatment (figure).31
Overall, Simponi® showed statistically higher persistence compared with other TNF inhibitors based on reported HR and p-values.31
Studies with Simponi® persistence data (HR) are illustrating that, where HR>1 the treatment persistence is in favour for Simponi® versus comparator.31
Sweden - Simponi® persistence data: Real World studies
n = 4903, AS : 21.0%, RA : 52.3%, year = 2016
Simponi® demonstrates higher persistence rates compared with adalimumab and etanercept when used in TNFi naïve IMRD patients, as reflected in a real-world study in Sweden in 4,903 patients.4
Simponi® had statistically significant higher persistence than ADA (p=0.022) and ETN (p=0.004) when comparing persistence rates over the full study period in all indications.4
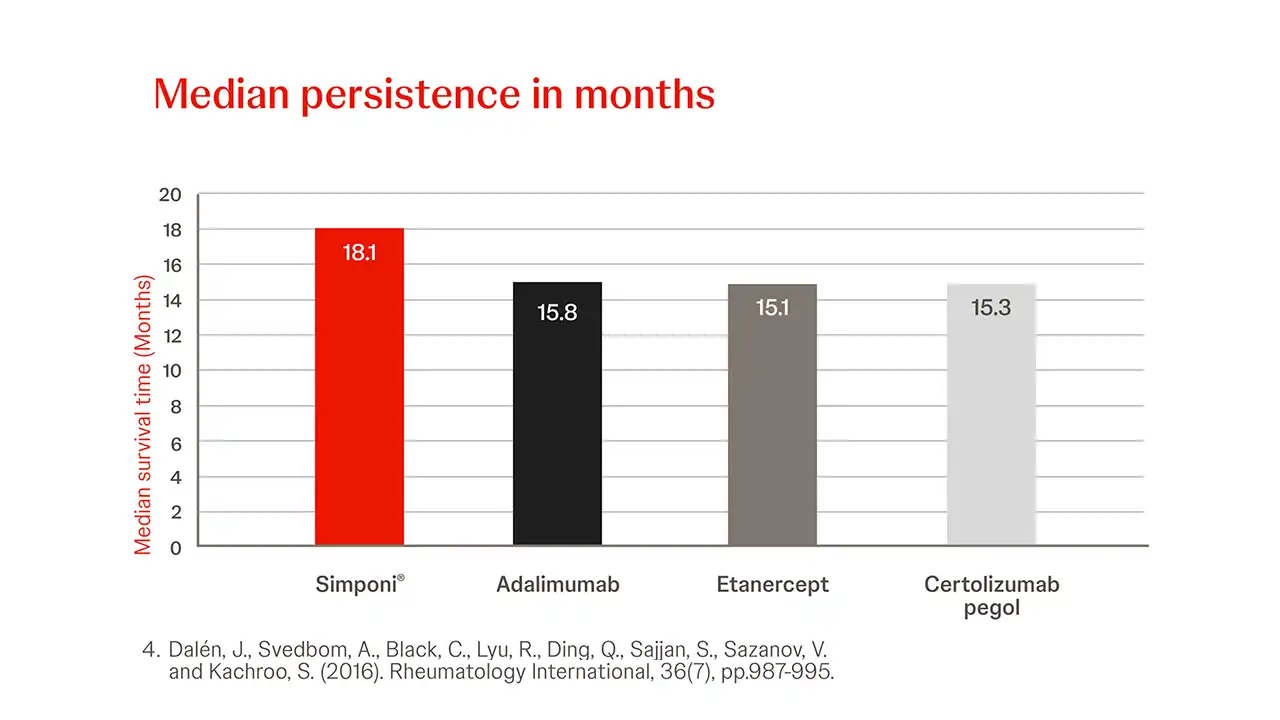
High Simponi® Treatment persistence newly treated with SC-TNFi4
In newly treated patients with IMRD compared to other SC-TNFi, Simponi® showed the longest treatment persistence duration.4
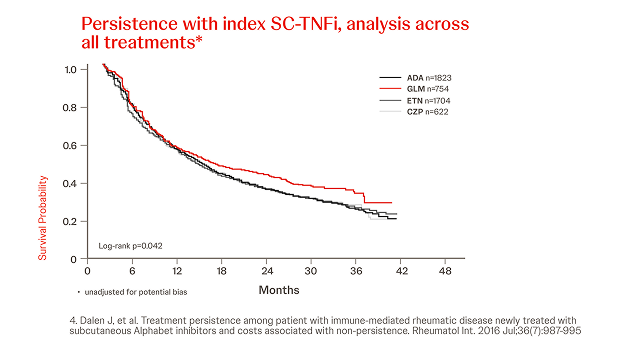
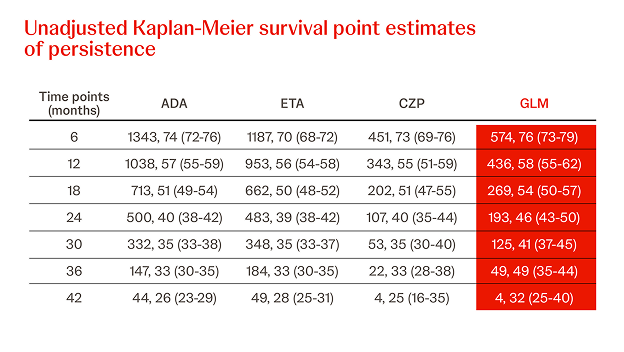
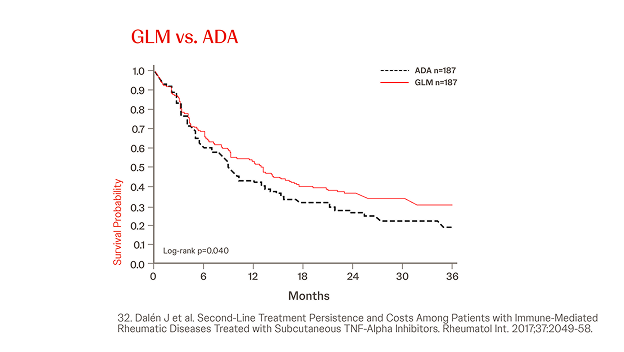
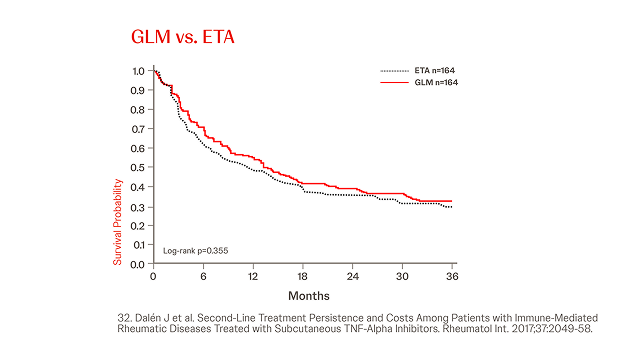
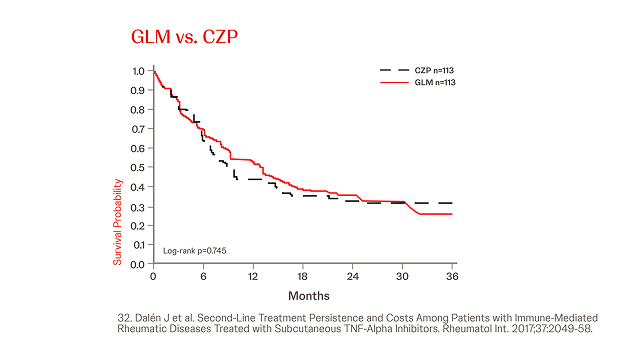
Propensity Score-matched Analyses of Persistence with 2nd-line SC-TNFi32
n = 845, year = 2017
In a different cohort IMRD patients treated with a subsequent line of biological therapy, in total, N = 845 patients were identified and three cohorts were generated (Simponi® vs. ADA, ETN, and CZP, respectively). Simponi® exhibited higher persistence than ADA over the study period (p = 0.040), and numerically higher persistence than ETN and CZP for 36 and 30 months, respectively (figure).32
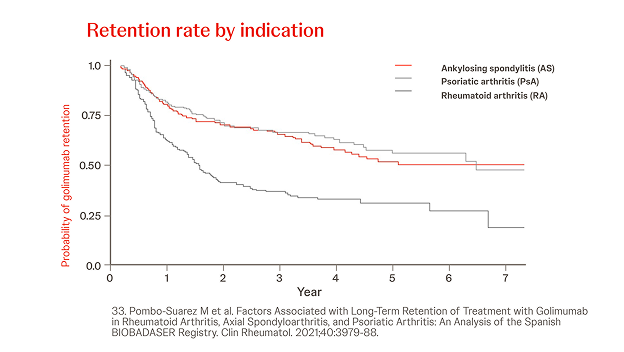
Spanish biological drugs registry BIOBADASER
n = 685, RA: 28.5%, AS: 42.9%, year = 2021
In a retrospective analysis among 685 patients (28.5% RA, 42.9% AS, 28.6% PsA), the overall probability of retention of Simponi® treatment was greater in patients with AS or PsA versus RA (p < 0.001) and when Simponi® was used as first-line treatment versus third or later lines (p < 0.001).33

Slovenian BioRx.si registry
n = 2022, year = 2019
In a prospective analysis of 2,022 patients treated with Simponi® or other TNFis (i.e. ADA, ETN, CZP, IFX) in patients with RA, AS and PsA, the persistence of Simponi® compared beneficially to other TNFis in bDMARD-experienced AS and PsA patients.23
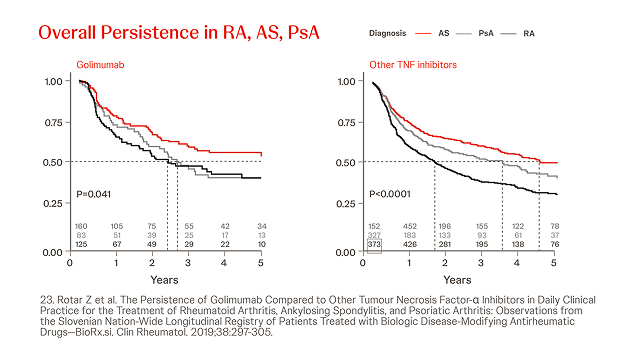
Slovenian assessment of treatment persistence stratified by treatment type, indication and prior exposure to bDMARDs. The crude and adjusted hazard ratios for Simponi® discontinuation did not differ significantly between bDMARD-naïve and bDMARD-experienced patients for any of the indications.23

Australian Data
In a retrospective cohort analysis conducted using the Australian Commonwealth Department of Human Services Pharmaceutical Benefits Scheme included data from 2,612 first-line patients and 1,276 second-line patients (RA, AS, PsA).35
Treatment discontinuation among first-line patients treated with etanercept or adalimumab was not significantly different from those treated with Simponi® (HR 1.10, 95% CI 0.95–1.28, P=0.22; HR 1.06, 95% CI 0.93–1.22, P=0.39; respectively).35
Among the 1,276 patients in the second-line cohort (etanercept=41%, adalimumab=41%, Simponi®=18%) discontinuation was significantly higher for patients on etanercept compared with Simponi® (HR 1.24, 95% CI 1.03–1.50, P=0.03).35

Greek data
n = 328, year = 2018
In a retrospective, observational study of 328 patients treated with Simponi® in 4 Academic Centres in Greece, Simponi® showed a high 3-year persistence in patients with inflammatory arthritides with a low rate of discontinuation due to AEs. AS patients treated with Simponi® showed the highest survival on drug (SOD) (72%) compared to the other two groups at the end of the follow-up period.36
Abbreviations
ADA: adalimumab; AS: radiographic Axial Spondyloarthritis; bDMARD: biological Disease Modifying Anti Rheumatic Drug; CI: Confidence Interval; CZP: certolizumab pegol; ETN: etanercept; GLM: Simponi® (golimumab); HR: Hazard Ratio; IMRD: Immune Mediated Rheumatic Diseases; PsA: Psoriatic Arthritis; RA: Rheumatoid Arthritis; RCTs: Randomized Controlled Trials; RWE: Real-World Evidence; SC-TNFi: Subcutaneous Tumor Necrosis Factor inhibitor; SOD: Survival on Drug; TNFi: Tumor Necrosis Factor inhibitor.
References
4. Dalen J, et al. Treatment persistence among patient with immune-mediated rheumatic disease newly treated with subcutaneous TNF-alpha inhibitors and costs associated with non-persistence. Rheumatol Int. 2016 Jul;36(7):987-995.
10. Aaltonen KJ et al. Efficacy and Effectiveness of Tumour Necrosis Factor Inhibitors in the Treatment of Rheumatoid Arthritis in Randomized Controlled Trials and Routine Clinical Practice. Rheumatology (Oxford). 2017;56(5):725-735. doi:10.1093/rheumatology/kew467.
13. Svedbom A, et al. Persistence with Golimumab in immune-mediated rheumatic diseases: a systematic review of real-world evidence in rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis. Patient Preference and Adherence 2017;11:719-729.
19. Bhoi P, Bessette L, Bell MJ et al. Adherence and Dosing Interval of Subcutaneous Antitumor Necrosis Factor Biologics Among Patients with Inflammatory Arthritis: Analysis from a Canadian Administrative Database. BMJ Open. 2017;7:e015872. doi:10.1136/bmjopen-2017-015872.
23. Rotar Z et al. The Persistence of Golimumab Compared to Other Tumour Necrosis Factor-α Inhibitors in Daily Clinical Practice for the Treatment of Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis: Observations from the Slovenian Nation-Wide Longitudinal Registry of Patients Treated with Biologic Disease-Modifying Antirheumatic Drugs—BioRx.si. Clin Rheumatol. 2019;38:297-305.
31. Luttropp K et al. Real-World Treatment Persistence of Golimumab in the Management of Immune-Mediated Rheumatic Diseases in Europe: A Systematic Literature Review. BMJ Open. 2019;9:e027456.
32. Dalén J et al. Second-Line Treatment Persistence and Costs Among Patients with Immune-Mediated Rheumatic Diseases Treated with Subcutaneous TNF-Alpha Inhibitors. Rheumatol Int. 2017;37:2049-58.
33. Pombo-Suarez M et al. Factors Associated with Long-Term Retention of Treatment with Golimumab in Rheumatoid Arthritis, Axial Spondyloarthritis, and Psoriatic Arthritis: An Analysis of the Spanish BIOBADASER Registry. Clin Rheumatol. 2021;40:3979-88.
35. Acar M et al. Treatment Persistence of Subcutaneous TNF Inhibitors Among Australian Patients with Immune-Mediated Rheumatic Disease (IMRD). Open Access Rheumatol Res Rev. 2018;10:151-160.
36. Thomas K et al. High 3-Year Golimumab Survival in Patients with Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis: Real-World Data from 328 Patients. Clin Exp Rheumatol. 2018;36:254-62.