Video Real World Evidence - RA
Watch this 1-minute video to explore real-world persistence of Simponi® in immune-mediated rheumatic diseases (IMRD) including RA as summarized from a systematic literature review.
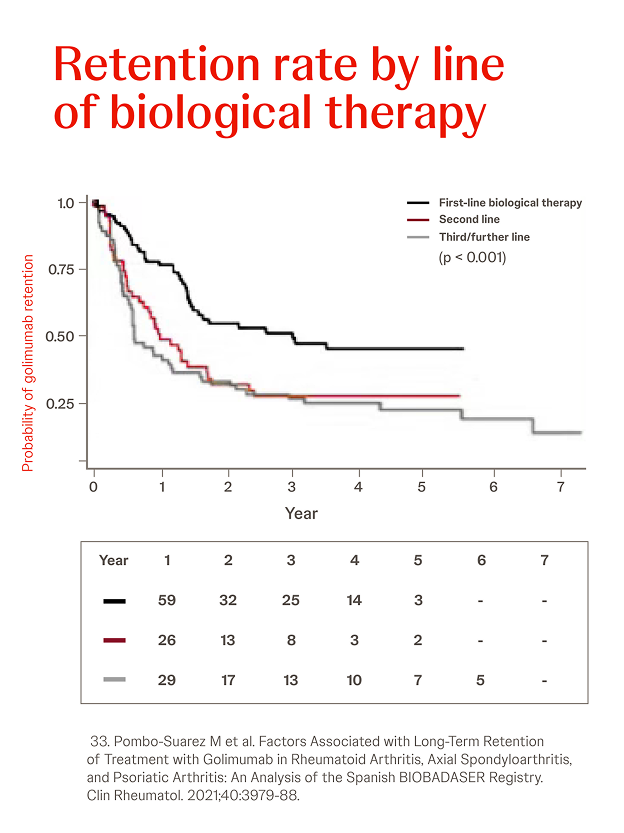
Spanish biological drugs registry BIOBADASER
n = 685, RA: 28.5%, AS: 42.9%, year = 2021
In a retrospective analysis among 685 patients (28.5% RA, 42.9% AS, 28.6% PsA), the overall probability of retention of Simponi® treatment was greater in patients with AS or PsA versus RA (p < 0.001) and when Simponi® was used as first-line treatment versus third or later lines (p < 0.001).33

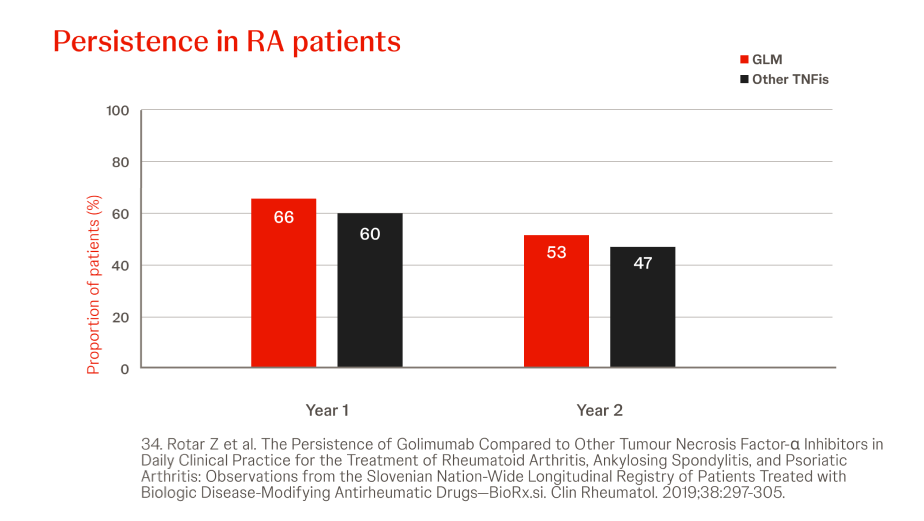
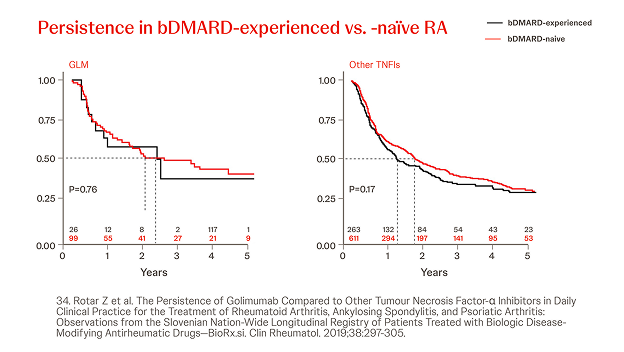
Slovenian BioRx.si registry
n = 2022, year = 2019
In a prospective analysis of RA patients treated with Simponi® (n=125) or other (n=874) TNFis (i.e. ADA, ETN, CZP, IFX), the persistence of Simponi® did not differ significantly from other TNFis at 2 years, though more patients were in DAS28ESR low-disease state (Simponi® vs other TNF: 50% vs 34% (p=0.0002)). Prior bDMARD exposure did not significantly influence the persistence of Simponi®.34
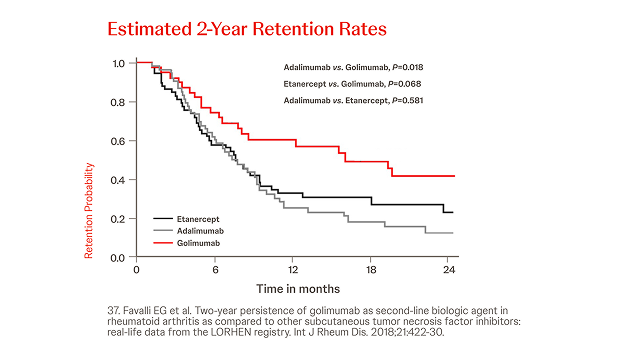
Italian LORHEN registry
n = 195, year = 2018
In an analysis on 195 RA patients treated with Simponi®, ETN or ADA, the 2-year retention rate was significantly lower for adalimumab (31.2%, P = 0.018) and numerically lower for etanercept (39.8%, P = 0.068) compared with Simponi® (53.4%) because of a higher discontinuation rate due to adverse events (P = 0.042 and P = 0.038 versus Simponi®, respectively).
These real-life data confirmed switching to a second TNFi as a good option for treating first-line TNFi failures in RA.37

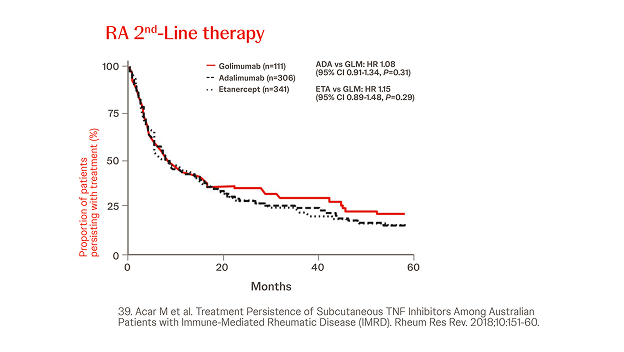
Australian Data
n = 2413, year = 2018
In a retrospective cohort analysis conducted using the Australian Commonwealth Department of Human Services Pharmaceutical Benefits Scheme included data from 2,413 RA patients. While persistence with ETN was significantly longer than with Simponi® in the third-line setting (HR 0.65, 95% CI 0.48–0.90, P=0.01), there were no other significant differences in persistence in first or second line therapy.39

Abbreviations
ADA: adalimumab; AS: radiographic Axial Spondyloarthritis; bDMARD: biological Disease Modifying Anti Rheumatic Drug; CI: Confidence Interval; CZP: certolizumab pegol; ETN: etanercept; GLM: Simponi® (golimumab); HR: Hazard Ratio; IMRD: Immune Mediated Rheumatic Diseases; PsA: Psoriatic Arthritis; RA: Rheumatoid Arthritis; RCTs: Randomized Controlled Trials; RWE: Real-World Evidence; SC-TNFi: Subcutaneous Tumor Necrosis Factor inhibitor; SOD: Survival on Drug; TNFi: Tumor Necrosis Factor inhibitor.
References
33. Pombo-Suarez M et al. Factors Associated with Long-Term Retention of Treatment with Golimumab in Rheumatoid Arthritis, Axial Spondyloarthritis, and Psoriatic Arthritis: An Analysis of the Spanish BIOBADASER Registry. Clin Rheumatol. 2021;40:3979-88.
34. Rotar Z et al. The Persistence of Golimumab Compared to Other Tumour Necrosis Factor- Inhibitors in Daily Clinical Practice for the Treatment of Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis: Observations from the Slovenian Nation-Wide Longitudinal Registry of Patients Treated with Biologic Disease-Modifying Antirheumatic Drugs—BioRx.si. Clin Rheumatol. 2019;38:297-305.
37. Favalli EG et al. Two-year persistence of golimumab as second-line biologic agent in rheumatoid arthritis as compared to other subcutaneous tumor necrosis factor inhibitors: real-life data from the LORHEN registry. Int J Rheum Dis. 2018;21:422-30.
38. Santoleri F et al. ADA_ETA_BIO2021: real-world evaluation of adherence, persistence, and cost-effectiveness of originator and biosimilar biological drugs in the treatment of rheumatoid arthritis: a multicenter study in Italy. Curr Med Res Opin. 2023;39:1729-35.
39. Acar M et al. Treatment Persistence of Subcutaneous TNF Inhibitors Among Australian Patients with Immune-Mediated Rheumatic Disease (IMRD). Rheum Res Rev. 2018;10:151-60.